For centuries, the universe’s most elusive secrets have fascinated scientists and laypeople alike, stirring curiosity about the very fabric of cosmic existence. Recent revelations about methanetetrol—a molecule once exclusively theorized—deliver a disruptive punch to our conventional understanding of interstellar chemistry. This discovery isn’t merely a scientific milestone; it’s a wake-up call that the universe holds far more complex, unpredictable chemical marvels than previously imagined.
Instead of bolstering the long-held belief that space is a barren, sparse environment with only simple molecules, this finding hints at an intricate, dynamic chemical ecosystem thriving in the depths of cold interstellar clouds. What else could have been dismissed as impossible or improbable but now demands reconsideration? The implications challenge every fundamental assumption about the limitations of space chemistry and open a Pandora’s box of potentially transformative scientific pursuits.
The False Comfort of Simplicity in Space Chemistry
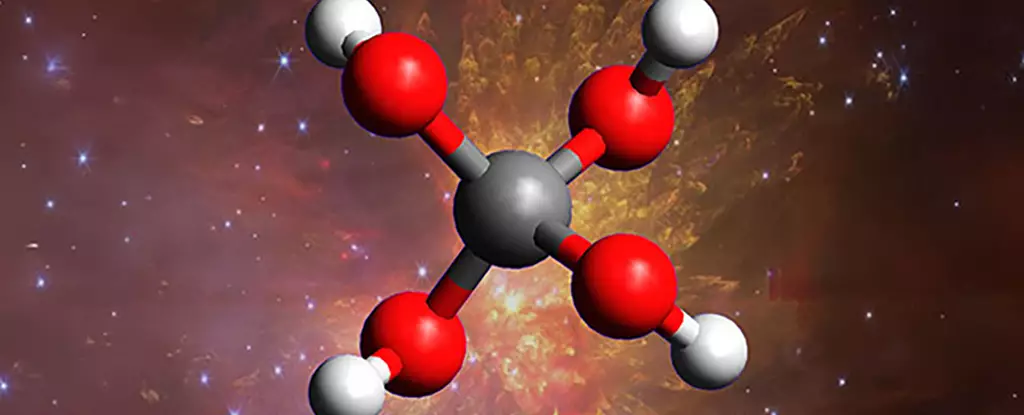
Much of our current understanding of molecules in space relies on assumptions rooted in terrestrial chemistry. We expect molecules to resemble their Earth-bound counterparts, ignoring the wild, extreme conditions that redefine chemical stability. The detection of methanetetrol, a highly unstable “super-alcohol” with unique four-hydroxyl groups attached to a single carbon, shatters this misconception. Its existence was long predicted but never observed until now, revealing that space’s environment fosters reactions that are impossible on Earth.
Scientists recreating space-like conditions in labs demonstrated that such molecules could form amid super cold temperatures—close to absolute zero—when subjected to cosmic-like radiation. The breakthrough exposes how space’s brutal environment, with high-energy radiation bouncing off ice particles and dust, catalyzes chemical processes that defy terrestrial intuition. We’ve been naive to believe that instability equates to impossibility; instead, space seems to be an extraordinary cauldron of fleeting, yet potentially significant molecules.
The Narrow Window to Natural Detection
Despite the excitement surrounding the creation of methanetetrol in lab conditions, the real challenge lies ahead: detecting this molecule amidst the chaos and distance of space. Its fleeting lifespan, caused by dissociative photoionization—where light breaks molecules apart—means that direct observation might remain out of reach for now. Our telescopes and detection instruments must evolve rapidly to catch these transient signatures, which may only exist virtually in scale before disintegrating.
This underscores a broader critique: our current technological constraints limit our comprehension of cosmic chemistry. As powerful as recent advancements are, they still fall short of fully capturing the rich chemical tapestry woven into the universe. Yet, this challenge shouldn’t be discouraging. Instead, it must propel us to innovate and push the boundaries of observational technology, connecting the dots between laboratory simulations and cosmic realities.
Implications for the Search for Life and Our Cosmic Destiny
The potential presence of such complex molecules as methanetetrol vastly alters the narrative about the origins of life. If molecules once deemed impossible can form in the harsh cold of space, then the raw ingredients for life could be more widespread than we had assumed. This bolsters a middle-ground perspective in the debate over extraterrestrial life: while not granting naive optimism, it encourages an open-minded approach that recognizes the universe’s capacity for generating complexity.
Furthermore, these findings challenge the idea that space is a chemically barren wasteland. Instead, it’s a dynamic forge where conditions often defy Earth-based logic. For those of us advocating for responsible scientific progress, this underscores the importance of sustained, interdisciplinary research that respects the universe’s unpredictability. Recognizing that space chemistry is more layered and counterintuitive than we thought should inspire a more nuanced global conversation about our place in the cosmos and the potential for discovering life beyond Earth.
In sum, the recent breakthrough reveals that the universe’s chemical landscape is far richer—and more perplexing—than conventional wisdom suggests. It is a stark reminder that scientific humility and curiosity must go hand-in-hand, especially as we venture further into the uncharted territories of space’s chemical mysteries.
Leave a Reply